Statement for the Record by Michael Kades for the House Committee on Oversight and Reform
Michael Kades
Director, Markets and Competition Policy
Washington Center for Equitable Growth
Statement for the Record
House Committee on Oversight and Reform
“Unsustainable Drug Prices (Part III): Testimony from AbbVie CEO”
May 18, 2021
This committee has done important work investigating prescription drug prices and the burden they place on both Americans’ pocketbooks and their health. As a former litigator and regulator with the Federal Trade Commission for almost 20 years, I hope to offer some historical context in which to understand the issues confronting the committee today.
Too often, too many pharmaceutical companies focus their innovation on new ways to delay competition, increase profits, and aggravate the burden on Americans struggling to pay their healthcare costs rather than on developing new lifesaving medications. AbbVie, whose CEO is testifying today, too often has been the posterchild for developing and employing anticompetitive practices that increase costs without any offsetting benefit.
Professor Craig Garthwaite, who is also testifying today, has correctly warned that there can be a trade-off between lowering cost and promoting innovation.1
These are hard questions that deserve careful consideration. But the type of anticompetitive conduct in which AbbVie has historically engaged represents the easy case. Congress can end these practices without any fear of deterring innovation.
AbbVie and pay-for-delay patent settlements
Before Humira and before Ibruvica, Solvay Pharmaceuticals—which AbbVie eventually acquired—protected its lucrative monopoly over Androgel, a testosterone replacement cream. Solvay paid not one, but two companies to refrain from selling their generic testosterone cream, in a practice known as a pay-for-delay patent settlement. The generic products would have taken 90 percent of branded Androgel’s sales at a substantial price discount. Although Solvay sued both companies for patent infringement, Solvay reached lucrative settlements with each generic competitor. The generic companies agreed to keep their testosterone creams off the market until 2015. Until then, each company would market Solvay’s branded product and receive a royalty on the branded product’s sales. Instead of trying to take sales from the monopolist, as a competitor should, the two companies would be trying to increase them; Solvay was literally sharing its monopoly profits to deter competition.
In a seminal case, Federal Trade Commission v. Actavis, the Supreme Court held that this type of agreement could violate the antitrust laws. By this time, AbbVie owned Solvay and the product Androgel. AbbVie could have quickly settled the case and opened the market to competition. Instead, AbbVie chose to fight tooth-and-nail for roughly 6 years, only settling with the FTC on the eve of trial. That delay helped prevent competition, allowed AbbVie to extract additional monopoly rents from consumers, and gave it the time to effect the product hopping strategy discussed below. The lesson here is that too many pharmaceutical companies use delay in the legal process to avoid judgement, increasing the effectiveness of their anticompetitive conduct.
AbbVie and frivolous patent litigation
By 2011, AbbVie faced a new competitive threat. Perrigo, a third generic company, filed an application to sell generic Androgel. Solvay itself had reached this conclusion and chosen not to sue Perrigo. AbbVie, which had acquired Solvay, ignored the merits. It understood that the time it would take Perrigo to win the patent case would keep the generic company off market for years and extend AbbVie’s Androgel monopoly.
The FTC brought an antitrust case against AbbVie. Antitrust cases based on sham litigation are somewhat of a unicorn because the legal standards are so high. The plaintiff must prove that “the lawsuit must be objectively baseless in the sense that no reasonable litigant could realistically expect success on the merits” and that “the baseless lawsuit conceals an attempt to interfere directly with the business relationships of a competitor . . . through the use [of] the governmental process—as opposed to the outcome of that process—as an anticompetitive weapon.”2
Despite those high standards, the District Court ruled in the FTC’s favor. Indeed, it found it indisputable that AbbVie’s case was objectively baseless—so frivolous that the court, in an antitrust case, did not need to hold an evidentiary hearing on the issue.3The court further found that the FTC had satisfied its burden on the second element as well.4 Finally, it ordered AbbVie to give up almost half a billion dollars that it earned by filing a sham lawsuit that delayed competition.5
The case has, however, an unfortunate ending. Although the Court of Appeals upheld the liability determination, it decided that the FTC could not deprive AbbVie of its illegal profits. In an unrelated case, the Supreme Court recently stripped the FTC entirely of its powers to seek monetary remedies like the one described here.
In short, AbbVie filed frivolous litigation, the very process of that litigation—not its outcome—delayed competition, that delay increased its profits by hundreds of millions of dollars—and AbbVie suffered no consequences.
AbbVie and product hopping
Neither the pay-for-delay settlement nor the frivolous litigation were isolated strategies. AbbVie had a larger plan to move patients from the original formulation of Androgel to a new version, a strategy referred to as product hopping.
New Androgel was not significantly better than the original, but it would blunt competition from the generic version of the original Androgel. AbbVie’s combined strategies delayed competition from generic versions of original Androgel until 2014, and AbbVie was able to convert 83 percent of the market before a generic entered. Once generic versions of the original version entered, however, new Androgel ceased gaining market share.
The pay for delay settlement and the sham litigation gave AbbVie additional years to move the market to the new, but not better, product.6 Had generic versions of the original product been available, consumers would have saved hundreds of millions of dollars by using the less expensive but equally effective generic alternative.
Implications for the Oversight hearing
Long ago, AbbVie moved on from Androgel, but this committee should not ignore the lessons of history. While strategies such as patent thickets may look different, they may be complex variations on a theme. For example, Humira’s original patents were set to expire in 2016. As that date approached, however, AbbVie began seeking a multitude of new patents for what seems to be minor improvements at best, but which have secured its monopoly for years and perhaps a decade or two.7 This committee should be skeptical of claims that every strategy that extends a company’s monopoly is necessary to protect innovation; too many are simply anticompetitive tactics to prevent legitimate competition.
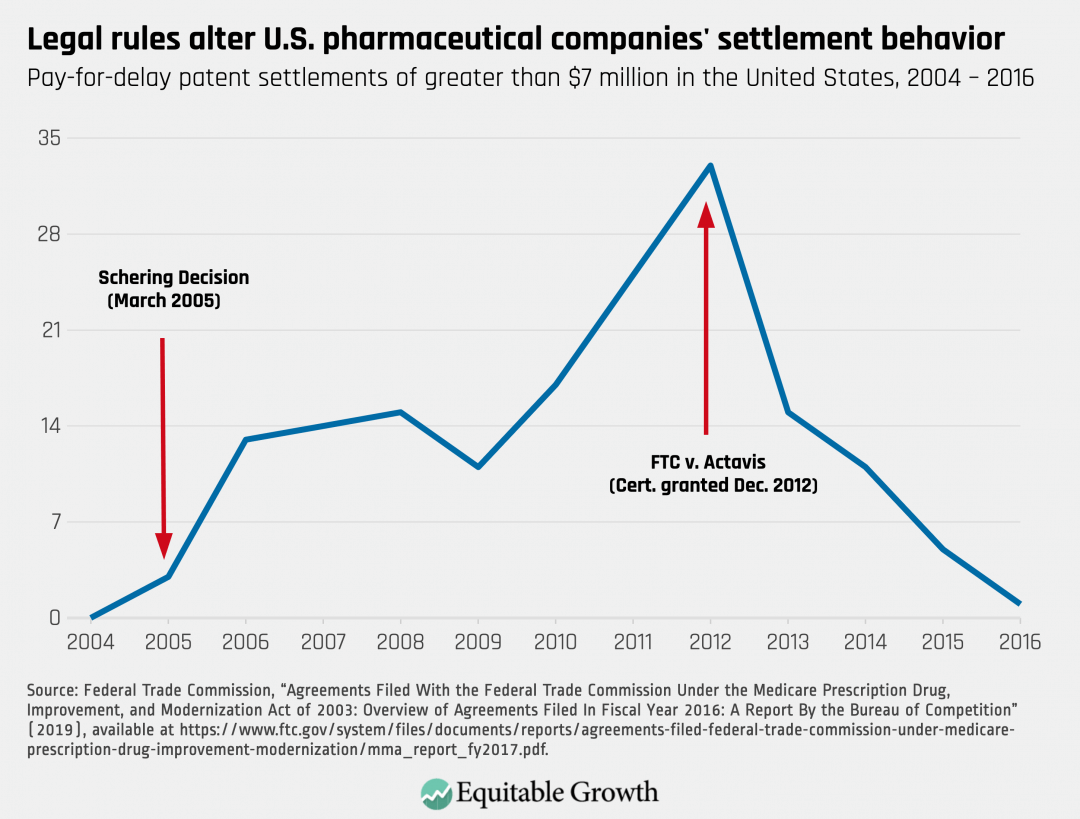
On the bright side, Congress can change market dynamics. Pharmaceutical companies respond to rules and incentives. For example, federal courts, beginning in 2005, took a very lenient view of pay-for-delay patent settlements. In response, pharmaceutical companies increasingly entered into them. When, however, the Supreme Court rejected that approach, pharmaceutical companies largely abandoned this practice. (See Figure 1.)
Figure 1
Similarly, for years, some branded companies denied generic companies sample products that the generic manufacturers needed to obtain regulatory approval from the Food and Drug Administration, while other branded companies refused to negotiate required safety protocols with the generic companies. According to the U.S. Food and Drug Administration, these practices were increasing prescription drug costs by $13.4 billion per year.8 In December 2019, Congress passed the CREATES Act to stop these practices. Both the Food and Drug Administration and the industry believe the bill has largely been successful.9
There are three proposals that Congress could adopt to limit anticompetitive conduct in the pharmaceutical industry.
- Stop pay-for-delay agreements: Despite the U.S. Supreme Court’s clear signal in the Actavis case that pay-for-delay settlements can be anticompetitive, the Federal Trade Commission continues to spend substantial resources and time challenging clear violations. Tougher laws, such as the Protecting Consumer Access to Generic Drugs Act10 or the Preserve Access to Affordable Generics Act,11 would deter such conduct and free up limited resources to attack other anticompetitive conduct.
- Restore the Federal Trade Commission’s disgorgement authority: A relatively simple modification to the Federal Trade Commission Act would clarify the FTC’s authority to deprive companies of any illegal profits they earned—authority that is critical to deterring highly profitable but anticompetitive conduct. Something is wrong when courts decide there are no repercussions for violating the antitrust laws.
- Deter strategic behavior such as product hopping and patent thickets when done to protect monopolies: By modifying patent law, the antitrust laws, and FDA law, Congress can likely deter purely rent-seeking activity that harms consumers without undermining legitimate incentives to innovate new and better pharmaceutical products.
Conclusion
This committee’s investigation is critical to understanding the scope of the problems that unnecessarily increase prescription drug costs. My statement has offered some historical context to understand today’s issues. Abbvie’s history of preventing competition through weak patents and frivolous lawsuits should should inform this Committee investigation into Abbvie’s current practices with regard to Humira and Imbruvia. I would be happy to work with the committee in any way to help it complete its important work.
End Notes
1. Craig Garthwaite and Benedic Ippolito, “Drug pricing conversations must take the cost of innovation into consideration,” STAT News, January 11, 2019, available at https://www.statnews.com/2019/01/11/drug-pricing-conversations-include-cost-innovation/.
2. Professional Real Estate Investors, Inc. v. Columbia Pictures Industries, Inc. 508 U.S. 49, 61 (1993), available at https://supreme.justia.com/cases/federal/us/508/49/.
3. In legal terms, the court found there was no factual dispute and granted judgment to the Federal Trade Commission on this issue.
4. AbbVie continues to challenge the case, seeking Supreme Court review; however, it is not challenging the finding that its patent case was objectively baseless. Instead, its position appears to be that it subjectively believed it could win the case. In other words, according to AbbVie, it and its lawyers were either incompetent or ignorant in understanding the merits of its case. See, generally, Petition for Writ of Certiorari, Abbvie v. Federal Trade Commission, available at https://www.supremecourt.gov/DocketPDF/20/20-1293/172095/20210316180937477_20-xxxx%20-%20AbbVie%20Inc.%20v.%20FTC%20-%20cert.%20petition.pdf.
5. Federal Trade Commission v. AbbVie Inc., 976 F.3d 327 (3rd Cir. 2020).
6. See generally, Federal Trade Commission v. Abbvie, 329 F. Supp. 3d 98, 142 (ED Pa. 2018), reviewed on other grounds Federal Trade Commission v. Abbvie Inc., 976 F.3d 327 (3rd Cir. 2020); Michael A. Carrier, “A real-world analysis of pharmaceutical settlements; the mission dimension of product hopping,” Florida Law Review 62 (2010).
7. Coalition Against Patent Abuse, “Abbvie’s Humira: The poster childe for how drug companies abuse the patent system to keep drug prices higher. But only for Americans” (n.d.), available at https://www.capanow.org/blog/abbvies-humira/.
8. Arnold Ventures, “REMS Abuse and the CREATES Act” (n.d.), available at https://www.scribd.com/document/420847142/REMS-Abuse-and-the-CREATES-Act#from_embed.
9. Office of Generic Drugs, “2020 Annual Report: Ensuring Access to Safe, Effective, High-quality, and More Affordable Generic Drugs” (Washington: Food and Drug Administration, 2021), p.7, available at https://www.fda.gov/drugs/generic-drugs/office-generic-drugs-2020-annual-report.
10. Protecting Consumer Access to Generic Drugs Act of 2021, H.R.153, 117th Cong. (2021), available at https://www.congress.gov/bill/117th-congress/house-bill/153.
11. Preserve Access to Affordable Generics and Biosimilars Act, S.64, 116th Cong. (2019), available at https://www.congress.gov/bill/116th-congress/senate-bill/64/text.